How to Use an Autoclave
There are a lot of products that need to be sterilized: pharmaceuticals, equipment, solutions, medical waste, and even tattoo needles! We use a…
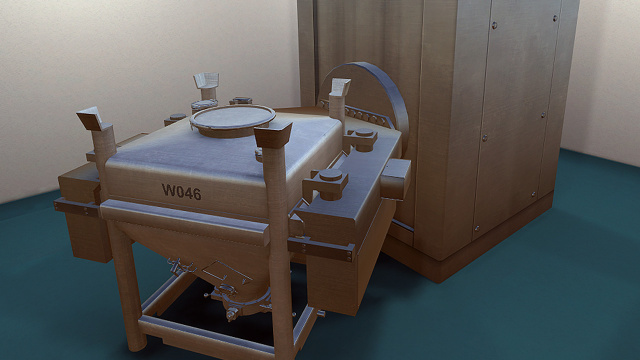
IBC Blender
This interactive lesson covers an overview of the IBC Blender hardware, a review of safety considerations, and interactive portions that include a…
Identifying Unknown Substances with Merck and the NC Crime Lab
How do scientists identify unknown substances collected as evidence at crime scenes? Learn about the process of investigating chemical and…
Immunology at MedTox
An industry leader in the development and validation of medical testing, LabCorp’s laboratories are internationally known as incubators for…
Improving Lives
Let's talk about some ways that biotechnology improves the lives of people. Here are just a few examples of life saving products made in North…
Introduction to Clinical Research
This series of activities explores the processes, research trials, and other considerations for developing and delivering a new drug product to the…
Introduction to Clinical Research: Part 1 – Drug Development Overview
This course provides a general overview of the process used to develop drugs and why it costs so much to bring those drugs to market. It also…
Introduction to Clinical Research: Part 2 – Human Subjects Protection and Informed Consent
Examine the reasons why there are protections for human participants in research studies and how permissions are collected from the candidates. Then…
Introduction to Clinical Research: Part 3 – Regulations and Guidance
Explore the federal and international regulations that protect human participants in research studies. Then use what you’ve learned to conduct…
Introduction to Clinical Research: Part 4 – Institutional Review Board (IRB)
Learn more about the Belmont Report as the foundation for the Institutional Review Board, an overview of the levels of review, and the use of consent…
Pagination
- First page
- Previous page
- …
- 9
- 10
- 11
- …
- Next page
- Last page