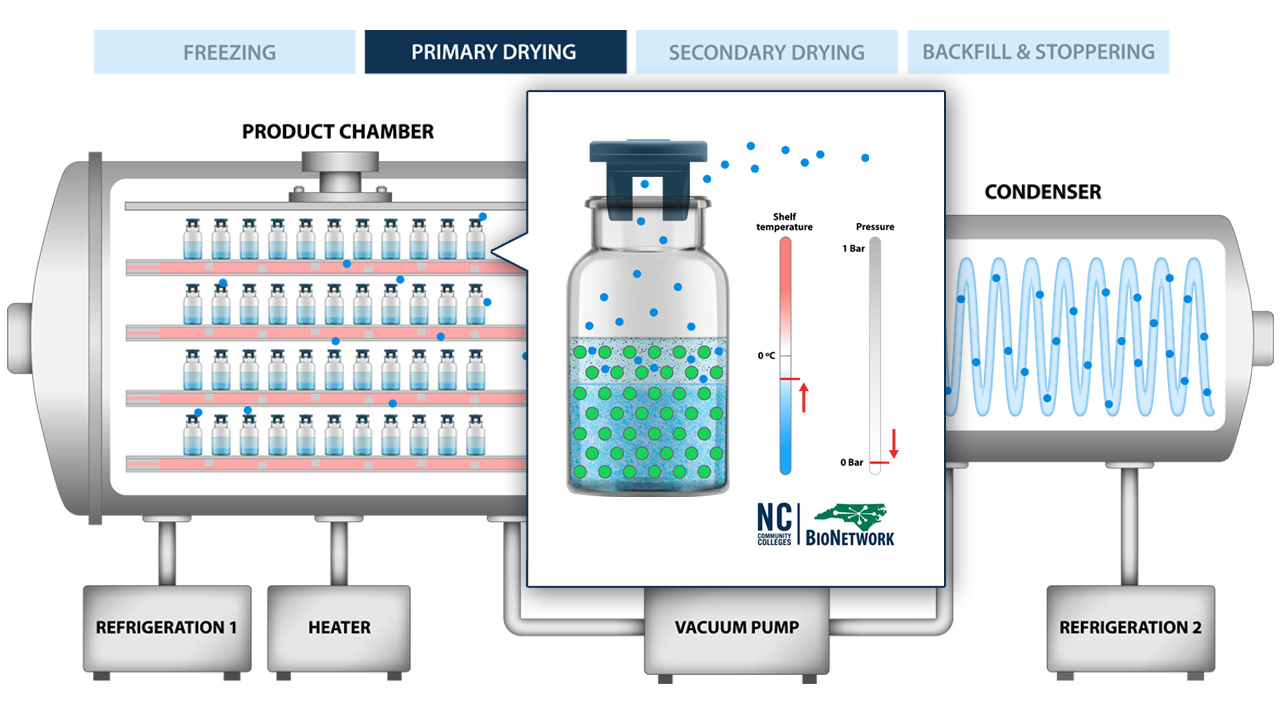
Lyophilization, or freeze drying, is the process of removing water from drug products to ensure long-term stability. Check out our 3 on-demand eCourses to learn more.
Lyophilization 1: Intro to Freeze Drying
Welcome to BioNetwork's introductory course on pharmaceutical lyophilization. Whether you're a community college student or a biomanufacturing…
Lyophilization 2: Freeze Drying Regulations and Validation
Welcome to BioNetwork’s second training course for freeze drying. In the next 20 minutes, we’ll examine important FDA regulations related to freeze…
Lyophilization 3: Freeze Drying Formulation and Cycle Development
Welcome to BioNetwork’s third lyophilization course. In this 20-minute course, we’ll explore the critical aspects of formulation development and…
The Process of Freeze Drying (Lyophilization)
Discover the science behind pharmaceutical freeze drying in this educational animation. Freeze drying, or lyophilization, is the process of removing…
Lyophilization
Lyophilization, or freeze drying, is an important process to the biopharmaceutical industry. Operate a virtual lyophilizer and explore the process of…